![]()
Muscle is a specialized contractile tissue that is a distinguishing characteristic of animals. Changes in muscle length support an exquisite array of animal movements, from the dexterity of octopus tentacles and peristaltic waves of Aplysia feet to the precise coordination of linebackers and ballerinas. What molecular mechanisms give rise to muscle contraction? The process of contraction has several key steps, which have been conserved during evolution across the majority of animals
When muscle cells are viewed under the microscope, one can see that they contain a striped pattern (striations). This pattern is formed by a series of basic units called sarcomeres that are arranged in a stacked pattern throughout muscle tissue (Figure 1). There can be thousands of sarcomeres within a single muscle cell. Sarcomeres are highly stereotyped and are repeated throughout muscle cells, and the proteins within them can change in length, which causes the overall length of a muscle to change. An individual sarcomere contains many parallel actin (thin) and myosin (thick) filaments. The interaction of myosin and actin proteins is at the core of our current understanding of sarcomere shortening. How does this shortening happen? It has something to do with a sliding interaction between actin and myosin.

The view of a mouse gastrocnemius (calf) muscle under a microscope. The sarcomeres are artifically colored green, and appear as stacked horzontal stripes of similar lengths. (White scale bar = 25 microns.)
![]()
© 2008 Nature Publishing Group Llewellyn, M. E. et al. Minimally invasive high-speed imaging of sarcomere contractile dynamics in mice and humans. Nature 454, 784-788 (2008). All rights reserved.
In 1954, scientists published two groundbreaking papers describing the molecular basis of muscle contraction. These papers described the position of myosin and actin filaments at various stages of contraction in muscle fibers and proposed how this interaction produced contractile force. Using high-resolution microscopy, A. F. Huxley and R. Niedergerke (1954) and H. E. Huxley and J. Hanson (1954) observed changes in the sarcomeres as muscle tissue shortened. They observed that one zone of the repeated sarcomere arrangement, the "A band," remained relatively constant in length during contraction (Figure 2A). The A band contains thick filaments of myosin, which suggested that the myosin filaments remained central and constant in length while other regions of the sarcomere shortened. The investigators noted that the "I band," rich in thinner filaments made of actin, changed its length along with the sarcomere. These observations led them to propose the sliding filament theory, which states that the sliding of actin past myosin generates muscle tension. Because actin is tethered to structures located at the lateral ends of each sarcomere called z discs or "z bands," any shortening of the actin filament length would result in a shortening of the sarcomere and thus the muscle. This theory has remained impressively intact (Figure 2B).
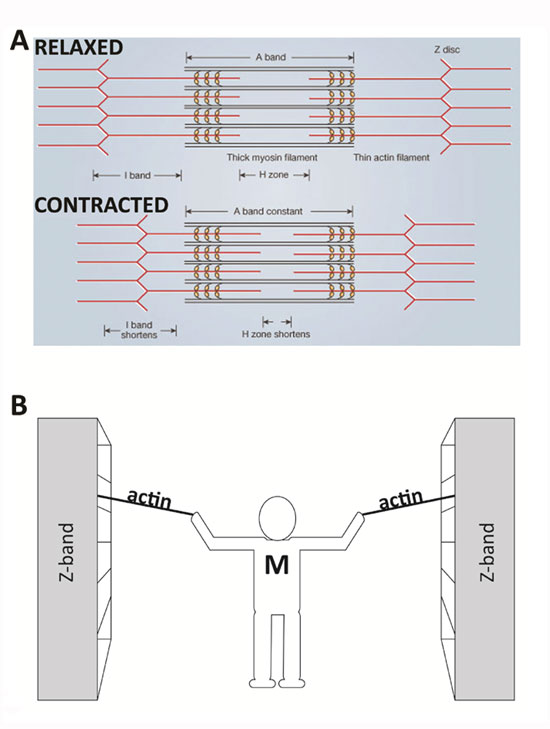
(A) The basic organization of a sarcomere subregion, showing the centralized location of myosin (A band). Actin and the z discs are shown in red. (B) A conceptual diagram representing the connectivity of molecules within a sarcomere. A person standing between two bookcases (z bands) pulls them in via ropes (actin). Myosin (M) is analogous to the person and the pulling arms. (z bands are also called z discs.)
![]()
© Nature Publishing Group A) adapted from Huxley, A. F. & Niedergerke, R. Structural Changes in Muscle During Contraction: Interference Microscopy of Living Muscle Fibres. Nature 172, 971-973 (1954). B) © Nature Education All rights reserved.
Imagine that you are standing between two large bookcases loaded with books. These large bookcases are several meters apart and are positioned on rails so that they can be easily moved. You are given the task of bringing the two bookcases together, but you are limited to using only your arms and two ropes. Standing centered between the bookcases, you pull on the two ropes — one per arm — which are tied securely to each bookcase. In a repetitive fashion, you pull each rope toward you, regrasp it, and then pull again. Eventually, as you progress through the length of rope, the bookcases move together and approach you. In this example, your arms are similar to the myosin molecules, the ropes are the actin filaments, and the bookcases are the z discs to which the actin is secured, which make up the lateral ends of a sarcomere. Similar to the way you would remain centered between the bookcases, the myosin filaments remain centered during normal muscle contraction (Figure 2B).
One important refinement of the sliding filament theory involved the particular way in which myosin is able to pull upon actin to shorten the sarcomere. Scientists have demonstrated that the globular end of each myosin protein that is nearest actin, called the S1 region, has multiple hinged segments, which can bend and facilitate contraction (Hynes et al. 1987; Spudich 2001). The bending of the myosin S1 region helps explain the way that myosin moves or "walks" along actin. The slimmer and typically longer "tail" region of myosin (S2) also exhibits flexibility, and it rotates in concert with the S1 contraction (Figure 3A).
The movements of myosin appear to be a kind of molecular dance. The myosin reaches forward, binds to actin, contracts, releases actin, and then reaches forward again to bind actin in a new cycle. This process is known as myosin-actin cycling. As the myosin S1 segment binds and releases actin, it forms what are called cross bridges, which extend from the thick myosin filaments to the thin actin filaments. The contraction of myosin's S1 region is called the power stroke (Figure 3). The power stroke requires the hydrolysis of ATP , which breaks a high-energy phosphate bond to release energy.

Actin (red) interacts with myosin, shown in globular form (pink) and a filament form (black line). The model shown is that of H. E. Huxley, modified to indicate bending (curved arrow) near the middle of the elongated cross bridge (subfragment 1, or S1) which provides the working stroke. This bending propels actin to the right approximately 10 nanometers (10 nm step). S2 tethers globular myosin to the thick filament (horizontal yellow line), which stays in place while the actin filament moves. Modified from Spudich (2001).
![]()
© 2001 Nature Publishing Group Spudich, J. A. The myosin swinging cross-bridge model. Nature Reviews Molecular Cell Biology 2, 387-392 (2001). All rights reserved.
Specifically, this ATP hydrolysis provides the energy for myosin to go through this cycling: to release actin, change its conformation , contract, and repeat the process again (Figure 4). Myosin would remain bound to actin indefinitely — causing the stiffness of rigor mortis — if new ATP molecules were not available (Lorand 1953).
Two key aspects of myosin-actin cycling use the energy made available by the hydrolysis of ATP. First, the action of the reaching myosin S1 head uses the energy released after the ATP molecule is broken into ADP and phosphate. Myosin binds actin in this extended conformation. Second, the release of the phosphate empowers the contraction of the myosin S1 region (Figure 4).
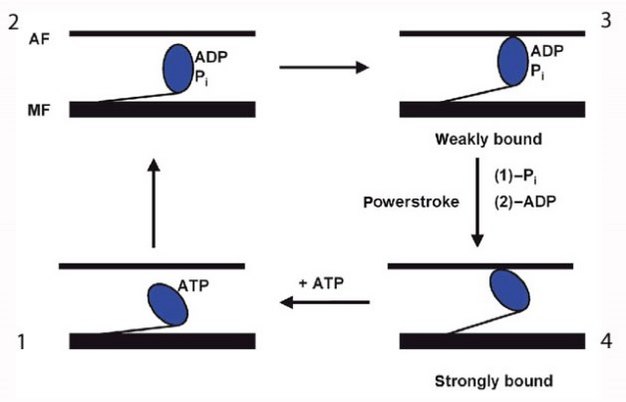 black line above and parallel to the myosin filament. A set of four arrows represents the transitions between each of the four steps." />
black line above and parallel to the myosin filament. A set of four arrows represents the transitions between each of the four steps." />
Figure 4: Illustration of the cycle of changes in myosin shape during cross-bridge cycling (1, 2, 3, and 4)
ATP hydrolysis releases the energy required for myosin to do its job. AF: actin filament; MF myosin filament. Modified from Goody (2003).
![]()
© 2003 Nature Publishing Group Goody, R. S. The missing link in the muscle cross-bridge cycle. Nature Structural Biology 10, 773-775 (2003). All rights reserved.
Calcium and ATP are cofactors (nonprotein components of enzymes) required for the contraction of muscle cells. ATP supplies the energy, as described above, but what does calcium do? Calcium is required by two proteins, troponin and tropomyosin, that regulate muscle contraction by blocking the binding of myosin to filamentous actin. In a resting sarcomere, tropomyosin blocks the binding of myosin to actin. In the above analogy of pulling shelves, tropomyosin would get in the way of your hand as it tried to hold the actin rope. For myosin to bind actin, tropomyosin must rotate around the actin filaments to expose the myosin-binding sites. In 1994, William Lehman and his colleagues demonstrated how tropomyosin rotates by studying the shape of actin and myosin in either calcium-rich solutions or solutions containing low calcium (Lehman, Craig, & Vibertt 1994). By comparing the action of troponin and tropomyosin under these two conditions, they found that the presence of calcium is essential for the contraction mechanism. Specifically, troponin (the smaller protein) shifts the position of tropomyosin and moves it away from the myosin-binding sites on actin, effectively unblocking the binding site (Figure 5). Once the myosin-binding sites are exposed, and if sufficient ATP is present, myosin binds to actin to begin cross-bridge cycling. Then the sarcomere shortens and the muscle contracts. In the absence of calcium, this binding does not occur, so the presence of free calcium is an important regulator of muscle contraction.

Simplified schematic of actin backbones, shown as gray chains of actin molecules (balls), covered with smooth tropomyosin filaments. Troponin is shown in red (subunits not distinguished). Upon binding calcium, troponin moves tropomyosin away from the myosin-binding sites on actin (bottom), effectively unblocking it. Modified from Lehman et al. (1994).
![]()
© 1994 Nature Publishing Group Lehman, W., Craig, R. & Vibert, P. Ca 2+ -induced tropomyosin movement in Limulus thin filaments revealed by three-dimensional reconstruction. Nature 368, 65-67 (1994). All rights reserved.